- Home
- About Us
-
Products
.png)
-
Application
.png)
-
Blog
.png)
- Contact us

9 Things Need to Be Considered When Choosing Flame Retardant

Table of Contents
1. Compatibility: How to Choose a Flame Retardant Compatible with Resin
2. Impact on Resin Properties: Balancing Flame Retardancy and Mechanical Properties
3. Low Cost: Selecting Cost-Effective Flame Retardants
4. Durability: Flame Retardant’s Longevity and Compatibility with Resin
5. Temperature Matching: Choosing the Right Flame Retardant for Resin
6. Properties of Flame Retardants: Understanding Different Types of Flame Retardants
7. Main and Auxiliary Flame Retardants: How to Combine Flame Retardants for Optimal Performance
8. Synergistic Effects: How to Maximize Flame Retardant Performance Through Combination
9. Counteractions: Which Flame Retardants Should Not Be Mixed
1. Compatibility: How to Choose a Flame Retardant Compatible with Resin
When selecting a flame retardant, the primary factor to consider is its compatibility with the resin. A flame retardant needs to be well-dispersed and compatible with the resin to optimize its flame-retardant properties. For example, in a flame-retardant polypropylene (PP) system, adding 15 phr of DBDPO can achieve the same flame-retardant effect as adding 5 phr of octa-PBDE, as octa-PBDE exhibits better compatibility with PP. However, it’s important to note that both DBDPO and octa-PBDE are now on the RoHS restricted list, so environmental compliance is a crucial consideration.
The flame retardant must not only improve the flame resistance but also maintain a good balance with the resin. If the flame retardant doesn’t mix well or has poor dispersion in the resin, it can result in reduced effectiveness, regardless of the quantity used.
2. Impact on Resin Properties: Balancing Flame Retardancy and Mechanical Properties
Flame retardants, especially inorganic types like magnesium hydroxide (MDH) and aluminum hydroxide (ATH), typically require high loading amounts to meet stringent flame-retardant standards, such as UL-94 V0. However, this can negatively affect the resin’s mechanical properties. For example, in an EVA/LDPE formula, when the loading amount of MDH exceeds 80 phr, it may not disperse evenly, leading to aggregation, which creates defects in the material. This results in a significant reduction in the tensile properties of the material, especially under external stress.
Therefore, it is essential to strike a balance between flame retardant performance and mechanical strength when formulating plastic compounds. The aim is to select a flame retardant that enhances flame resistance without compromising the material's overall strength and performance.

3. Low Cost: Selecting Cost-Effective Flame Retardants
Cost is a significant consideration when choosing a flame retardant. The ideal flame retardant should offer good performance at an affordable price. For industries that require large quantities of flame retardant, such as in cables, automotive parts, or construction materials, choosing a cost-effective option can lead to substantial savings while maintaining product quality.
While price is important, it should not come at the expense of long-term performance. The selected flame retardant must not only be economical but also stable and effective over time, ensuring that the flame-retardant properties are maintained during the product’s lifecycle.
4. Durability: Flame Retardant’s Longevity and Compatibility with Resin
The durability of the flame retardant is another critical factor to consider. A flame retardant should be stable over time and should not migrate or evaporate too quickly from the resin matrix. If a flame retardant migrates too quickly, it may separate from the resin before it can effectively act on the material, leading to a loss of its flame-retardant properties.
For long-term stability, the flame retardant must be well-integrated with the resin and able to withstand environmental conditions such as high temperatures and UV exposure. This is particularly important in outdoor or high-temperature applications where flame retardants may degrade over time.

5. Temperature Matching: Choosing the Right Flame Retardant for Resin
The decomposition temperature of the flame retardant must match the temperature range of the resin during processing and in service. The decomposition temperature of the flame retardant should generally be at least 20°C higher than the processing temperature of the resin. This ensures that the flame retardant remains stable during the production process.
Moreover, the decomposition temperature of the flame retardant should be compatible with the thermal degradation temperature of the resin, typically 60°C lower than the resin’s thermal degradation temperature. This ensures that the resin will not degrade prematurely while the flame retardant remains effective.
6. Properties of Flame Retardants: Understanding Different Types of Flame Retardants
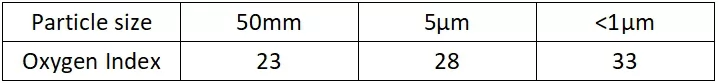
1. Impact of Ultrafine Particle Size
The particle size of a flame retardant has a significant effect on both flame retardancy and mechanical properties. For example, using ultrafine particles of ATH (Aluminum Trihydrate) can improve both flame retardancy and mechanical properties. When 80 phr of ATH is added to LDPE, the flame retardant performance improves as the particle size decreases. Smaller particles can better disperse within the resin, improving the overall effectiveness and reducing potential defects.
2. Application of Microencapsulation Technology
Microencapsulation involves coating the flame retardant particles with organic materials, polymers, oligomers, or inorganic substances. This technique can enhance the compatibility of the flame retardant with the resin, improving its dispersion and stability. Microencapsulation can also reduce the volatility of the flame retardant and increase its durability over time.
3. Surface Modification Techniques
Surface modification is particularly useful for inorganic flame retardants. By modifying the surface of the flame retardant particles with dispersing agents, plasticizers, or other additives, manufacturers can enhance compatibility and reduce the impact on the resin’s properties. This can lead to a better overall performance in terms of both flame retardancy and mechanical strength.
7. Main and Auxiliary Flame Retardants: How to Combine Flame Retardants for Optimal Performance
1. Main Flame Retardants
Main flame retardants are typically used in larger quantities and play the primary role in enhancing flame resistance. These include flame retardant resins, organic halogens, organic phosphorus compounds, and metal hydroxides. The loading amounts vary depending on the type of flame retardant; for example, organic flame retardants typically require 20 phr, while flame retardant resins require 30-40 phr, and metal hydroxides may require over 100 phr.
2. Auxiliary Flame Retardants
Auxiliary flame retardants are often used in smaller quantities and serve to enhance the overall performance of the main flame retardant. These include synergist flame retardants and smoke suppressants. Synergistic agents like antimony trioxide, zinc borate, and inorganic phosphorus compounds can significantly improve flame resistance when combined with other flame retardants. Smoke suppression agents, such as molybdenum compounds and iron compounds, can reduce the amount of smoke produced during combustion, improving safety.

8. Synergistic Effects: How to Maximize Flame Retardant Performance Through Combination
1. Halogen/Antimony Compounds
A common example of a synergistic combination is the use of halogenated flame retardants with antimony trioxide. Antimony oxide alone has minimal flame-retardant effect, but when combined with halogenated flame retardants, it creates a highly effective flame-retardant system. The optimal ratio of halogen to antimony is typically 3:1 for maximum synergy.
2. ATH/MDH Compound
Combining aluminum hydroxide (ATH) and magnesium hydroxide (MDH) can enhance their individual flame-retardant effects. In addition to their heat-absorbing properties and the water vapor generated during decomposition, aluminum hydroxide also promotes the carbonation of magnesium hydroxide, improving the overall flame retardant performance. The ideal ratio of these two is between 3:1 to 2:1.
3. Phosphorus-based Flame Retardants
Phosphorus-based flame retardants show synergistic effects with nitrogen-based flame retardants. Nitrogen can promote the carbonization of phosphorus compounds, enhancing their flame-retardant properties. For example, a mixture of 2% phosphorus and 2.5% nitrogen can be more effective than using phosphorus alone.
For the synergistic effect see the below equation.
3.5%P=2%P+2.5%N=1.4%P+4%N=0.9%P+5%N

9. Counteractions: Which Flame Retardants Should Not Be Mixed
Certain combinations of flame retardants can counteract each other, reducing their overall effectiveness. For example:
1. Halogenated Flame Retardants and Silicones
Halogenated flame retardants should not be used in combination with silicone-based flame retardants. When mixed, the oxygen index may decrease by 6-7%, reducing the effectiveness of both.
2. Red Phosphorus and Silicones
Red phosphorus should also be avoided in combinations with silicones. The interaction between these materials can result in a reduction of the oxygen index by more than 20%, undermining their flame-retardant performance.
3.Brominated Flame Retardants and Zinc Stearate
Zinc stearate and brominated flame retardants should not be mixed due to their chemical incompatibility. This combination may lead to undesirable effects such as material degradation or poor flame-retardant performance.
4. Calcium Carbonate and Magnesium Carbonate with Brominated Flame Retardants
Calcium and magnesium carbonate compounds should not be mixed with brominated flame retardants. The reaction between the two can reduce the flame retardant’s effectiveness and generate undesirable byproducts during processing.
Your Name*
Your Email*
-
2025-Oct-17Top 10 Aluminum Hydroxide Manufacturers in 2025Discover the top 10 aluminum hydroxide manufacturers in 2025, offering high-purity products for flame retardants, pharmaceuticals, and more.
-
2025-Oct-17Guide to Magnesium Hydroxide Flame Retardants for CablesLearn all about the key flame retardants used in the cable and wire industry, including magnesium hydroxide. This detailed guide covers the benefits, applications, and standards for flame retardant coatings. Stay ahead with sustainable and effective solutions from KMT Industry.
-
2025-Oct-17Top 10 Magnesium Hydroxide Suppliers in 2025Top 10 magnesium hydroxide manufacturers and suppliers leading the global market in 2025, detailed report on each supplier is provided.


-

 +86-931-7653361
+86-931-7653361 Room 1212, 1213, Jinhe Building, No. 1264 Beibinhe West Road, Anning District, Lanzhou City, Gansu Province, China.
Room 1212, 1213, Jinhe Building, No. 1264 Beibinhe West Road, Anning District, Lanzhou City, Gansu Province, China. -
Quick Links
-
Products